The U.S. Food and Drug Administration approved Amjevita, a drug similar to Humira, for use in the United States in 2016. This is a significant milestone for the pharmaceutical industry, as Amjevita is an important biosimilar to be approved in the United States. Biosimilars are drugs similar to existing biologic drugs and are expected to provide a more affordable option for patients. With the approval of Amjevita, the FDA has opened the door for more biosimilars to be approved in the future, potentially providing more affordable treatments for various diseases. This article will review all the details about these two drugs, including their similarities and differences. We’ll also talk about diseases they’re approved to treat, side effects, common myths, and the risks of taking these drugs during pregnancy. Read on to learn all this and more!
Table Of Contents
ToggleAbout Humira
Humira is a biologic medication used to treat various autoimmune diseases and is a monoclonal antibody, which means it is made from a single type of immune system cell. It works by binding to the target protein, preventing it from activating the body’s inflammatory response. This helps reduce inflammation and the symptoms associated with autoimmune diseases. Humira is generally used with other medications, such as methotrexate or corticosteroids. It is usually taken once a week, although the exact dosage and frequency may vary depending on the condition being treated. Common side effects of Humira include injection site reactions, upper respiratory infections, headaches, and abdominal pain. More severe side effects may include an increased risk of developing certain types of cancer and infections. Talking to your doctor about the potential risks and benefits of taking Humira before starting treatment is essential.
Diseases Humira is Approved to Treat
Humira is approved to treat a variety of chronic diseases. It is a type of drug called a tumor necrosis factor (TNF) blocker, which works by blocking the action of TNF, a protein that causes inflammation in the body. Humira is approved to treat various autoimmune diseases, including rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, Crohn’s disease, ulcerative colitis, and plaque psoriasis. For rheumatoid arthritis, Humira can reduce joint pain, swelling, and tenderness and help prevent further joint damage.
Amgevita (Adalimumab)
$371.13Humira (Adalimumab)
$2,150.00
It can also be combined with other medications to reduce the signs and symptoms of the disease. For psoriatic arthritis, Humira can reduce the joint pain and swelling associated with the condition and improve physical function. It can also be combined with other medications to reduce the signs and symptoms of the disease.
For ankylosing spondylitis, Humira can reduce the pain and inflammation associated with the condition and improve physical function. It can also be combined with other medications to reduce the signs and symptoms of the disease. For Crohn’s disease, Humira can reduce the inflammation associated with the condition and improve physical function. It can also be combined with other medications to reduce the signs and symptoms of the disease. For ulcerative colitis, Humira can reduce the inflammation associated with the condition and improve physical function. It can also be combined with other medications to reduce the signs and symptoms of the disease. For plaque psoriasis, Humira can reduce the itching, redness, and scaling associated with the condition and improve physical function. It can also be combined with other medications to reduce the signs and symptoms of the disease.
Humira is also used for Hidradenitis Suppurative (HS).
About Amjevita
Amjevita is a biological medication for adults’ with moderate to severe plaque psoriasis. It is a human monoclonal antibody that targets and blocks a protein called IL-23, which is involved in developing psoriasis. This helps reduce psoriasis symptoms, such as itching, scaling, and redness. Amjevita is administered as an injection under the skin, usually once a month. It is not recommended for people with an active infection, pregnant women, or people allergic to any ingredients in the medication. Common side effects of Amjevita include injection site reactions, upper respiratory tract infections, and headaches. Serious side effects, such as anaphylaxis or a severe allergic reaction, can occur in rare cases. Therefore, it is essential to discuss the potential risks and benefits of Amjevita with a healthcare provider before starting treatment. Nevertheless, Amjevita is a powerful and effective medication that can help people with moderate to severe plaque psoriasis manage their condition.
Diseases Amjevita is Approved to Treat
Amjevita is a biologic medication approved by the U.S. Food and Drug Administration (FDA) to treat certain autoimmune diseases. It is a type of monoclonal antibody, which is a laboratory-made protein that is designed to target and block a specific protein in the body. Amjevita is specifically designed to block the action of tumor necrosis factor-alpha (TNF-alpha), a protein that plays a role in inflammation. Amjevita helps reduce inflammation and its associated symptoms by blocking the action of TNF-alpha. Amjevita is approved to treat three autoimmune diseases: rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis.
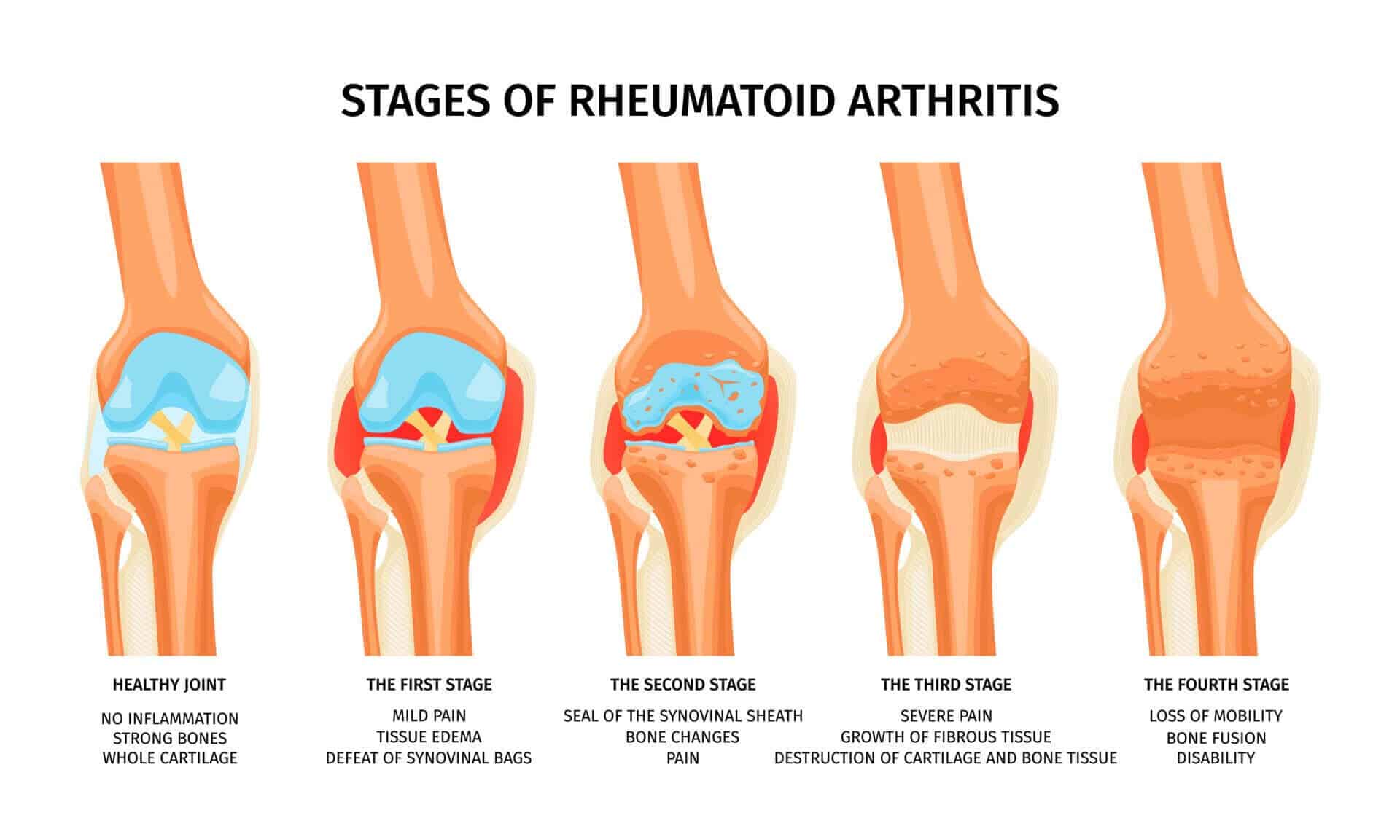
Rheumatoid arthritis is an autoimmune disorder that causes inflammation of the joints and can lead to joint damage and disability. Psoriatic arthritis is a type of arthritis that affects people with psoriasis, a skin condition characterized by red, scaly patches of skin. Ankylosing spondylitis is a form of arthritis that affects the spine and other body parts.
In addition to treating autoimmune diseases, Amjevita may also treat other conditions involving inflammation, such as Crohn’s disease, ulcerative colitis, and plaque psoriasis. However, it is essential to note that Amjevita is not approved to treat any of these conditions. Amjevita is typically administered as an injection under the skin. It is essential to follow your doctor’s instructions when taking Amjevita. Common side effects of Amjevita include injection site reactions, upper respiratory infections, and headaches. More severe side effects, such as severe infections and allergic reactions, are also possible. It is essential to talk to your doctor about any side effects you experience while taking Amjevita.
Similarities Between Humira and Amjevita
Humira and Amjevita are two drugs that have a lot of similarities. Biologic medications treat various autoimmune diseases, such as rheumatoid arthritis, Crohn’s disease, and plaque psoriasis. Both drugs target and block tumor necrosis factor (TNF), a protein in the body that can cause inflammation. By blocking this protein, Humira and Amjevita can help reduce inflammation and the symptoms associated with autoimmune diseases. Regarding safety, both drugs have been approved by the FDA and are considered safe and effective treatments for various autoimmune diseases. They are both administered via subcutaneous injection, which is injected into the skin. The injection site should be rotated to avoid irritation or infection.
Both drugs have similar side effects, such as nausea, headache, and injection site reactions. However, there are some differences in the more severe side effects. For example, Humira has been linked to an increased risk of certain types of infections, such as tuberculosis, while Amjevita has not been linked to such infections.
When it comes to cost, both drugs are expensive, but Humira is usually more expensive than Amjevita. The cost of Humira can range from $5,000 to $10,000 per year, while the cost of Amjevita is generally around $4,000 to $6,000 per year. Overall, Humira and Amjevita are two drugs with many similarities. They both target and block tumor necrosis factor, they are both considered to be safe and effective treatments for a variety of autoimmune diseases, they both have similar side effects, and they both have similar costs.
Many patients have purchased their Humira from Canada to ease up the cost of the medication. However, most healthcare coverage do cover Humira through their insurance plans.
Differences Between Humira and Amjevita
Humira and Amjevita are two drugs used to treat various chronic inflammatory diseases. Both medications are biological drugs, constituting proteins derived from living organisms.
Humira is a monoclonal antibody, while Amjevita is a biosimilar, meaning it is a copy of an existing biologic drug. Humira treats rheumatoid arthritis, Crohn’s disease, ulcerative colitis, psoriatic arthritis, ankylosing spondylitis, and plaque psoriasis. Amjevita, on the other hand, is approved to treat rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, and plaque psoriasis. Both medications are administered via subcutaneous injection. However, Humira is available in a pre-filled syringe and an auto-injector, while Amjevita is only in a pre-filled syringe. The active ingredient in Humira is adalimumab, while the active ingredient in Amjevita is adalimumab-atto. Adalimumab-atto is a slightly modified version of adalimumab and is designed to have a similar structure and function to adalimumab.
Before Taking
Before taking either Amjevita or Humira, it is essential to understand the potential risks associated with these medications.

It is crucial to speak with your doctor and discuss any potential risks, as well as any potential side effects that may occur. Additionally, it is essential to understand the potential interactions between these medications and other medications you may be taking. For example, suppose you are taking nonsteroidal anti-inflammatory drugs (NSAIDs) or aspirin. Discussing this with your doctor is vital as these medications may interact with Amjevita or Humira.
Furthermore, you must be aware of any potential allergies to these medications and any other allergies you may have.
Additionally, it is essential to discuss any potential risks associated with any medical conditions you may have, as these medications may not be suitable for specific conditions.
It is essential to discuss any potential lifestyle changes necessary while taking Amjevita or Humira, such as avoiding alcohol or smoking. By understanding the potential risks associated with these medications, you can make an informed decision about whether or not Amjevita or Humira is the right choice for you.
Misconceptions About These Drugs
Many misconceptions exist about Amjevita and Humira. One of the most common misconceptions is that these drugs are only used to treat severe cases of these diseases. In reality, both Amjevita and Humira can treat mild to moderate cases of these diseases and can even be used as preventative measures to reduce the risk of flare-ups. Another misconception is that these drugs are only for adults. While it is true that these drugs are not approved for use in children, they can be prescribed to adolescents over the age of 12.
Finally, some believe these drugs are unsafe and have serious side effects. While it is true that both drugs can have side effects, these side effects are usually mild and manageable. Both drugs have been approved by the FDA and are considered safe for use. In conclusion, there are many misconceptions about Amjevita and Humira. Understanding these drugs before starting treatment is essential, as they can be an effective and safe treatment option for many people.
Risks Of Pregnant Women Taking These Drugs
Pregnant women considering taking Amjevita or Humira should know the potential risks involved. Both medications are biologics, made from living organisms, and used to treat autoimmune diseases such as rheumatoid arthritis, psoriasis, and Crohn’s disease. While biologics can effectively treat these conditions, pregnant women should be aware of the potential risks of taking these medications.
The most common risks of taking Amjevita or Humira during pregnancy include an increased risk of miscarriage, preterm birth, or congenital disabilities. While the risk of these complications is generally low, pregnant women must discuss the potential risks with their healthcare provider before taking either medication. Additionally, it is essential to note that the safety and effectiveness of these medications during pregnancy have not been established.
Knowing the potential risks associated with breastfeeding while taking Amjevita or Humira is essential. While the amount of the drug passed to the baby through breast milk is typically low, discussing the risks with your healthcare provider before taking either medication while breastfeeding is essential. Pregnant women should know the potential risks of taking Amjevita or Humira during pregnancy or while breastfeeding. It is vital to discuss the risks with your healthcare provider before taking either medication, as the safety and effectiveness of these medications during pregnancy have not been established.
Conclusion
Overall, Humira and Amjevita are both very similar drugs, but also have some key differences. These drugs target and block specific proteins in the body that cause inflammation, and they are used to treat autoimmune diseases. Amjevita, however, is a biosimilar of Humira, meaning that it is essentially the same drug, but produced by a different manufacturer. Clinical trials have shown that the two drugs are equally effective, but their side effects and dosage schedules may differ slightly. A variety of factors, including cost, availability, and individual patient needs, will influence the decision between Humira and Amjevita. Choosing the best treatment option for a particular condition should be determined in collaboration with the patient’s healthcare provider.
Share: