The 2x Referral Bonus Event Is Now On! More Info ➡️
Home » Medication » Trelegy » Trelegy Ellipta
SAVE 10% OFF First Order With Coupon Code: WELCOME10

Our Price: $155.27 – $184.95

Trelegy Ellipta is a prescription medication dedicated to patients with COPD and asthma.
Buy Trelegy from Canada and save even $700 on your next purchase.
Step 1
Place Your Prescribed Trelegy Dosage In Your Cart
Step 2
Complete Checkout
Step 3
Upload Your Trelegy Prescription
(Shipping takes approximately 4-10 business days. A tracking number will be provided)
Trelegy Ellipta is a prescription medication used for the long-term, once-daily maintenance treatment of:
‼️Trelegy is not a rescue medication. It should not be used during an emergency, as it is a maintenance medication, not a fast-acting inhaler.
Trelegy Ellipta is a prescription medicine used to treat COPD – a chronic lung disease that includes chronic bronchitis, emphysema, or both.
Trelegy Ellipta is used to improve symptoms of COPD for better breathing and to reduce the number of flare-ups.
The medication can also be used to prevent and control asthma symptoms, such as wheezing, for better breathing.
🗒️Worth Noting
Vilanterol, a LABA, presents potential risks when used as a single asthma treatment. However, in Trelegy Ellipta, its combination with an inhaled corticosteroid and anticholinergic significantly reduces the likelihood of severe asthma outcomes.
Trelegy Ellipta contains three active ingredients that work together to help improve breathing:
This triple action helps to improve lung function, reduce symptoms such as shortness of breath and coughing, and enhance overall respiratory health.
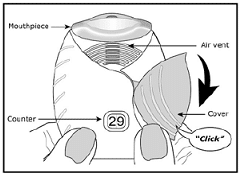
Read the step-by-step instructions for using this medication in the container. Do not use Trelegy Ellipta unless your healthcare provider has taught you how to use the inhaler and understand how to use it correctly. You can also call Insulin Outlet and speak to one of our friendly pharmacists.
Here’s a helpful step-by-step guide on how to use a Trelegy Ellipta inhaler:
Use Trelegy Ellipta at the same time each day.
Do not stop using Trelegy Ellipta unless told to do so by your healthcare provider because your symptoms might worsen. Your doctor will change your medicines as needed.
‼️Keep a fast-acting inhaler readily available for immediate symptom relief. If you lack a rescue inhaler, promptly contact your healthcare provider for a prescription. Seek immediate medical attention if your breathing deteriorates, your rescue inhaler use increases, or your symptoms persist despite using it.
Trelegy Ellipta comes in 2 different strengths:
Your healthcare provider prescribed the strength that is best for you. Use this medication exactly as your healthcare provider tells you to use it.
If you miss a dose of Trelegy Ellipta, take it as soon as you remember. Do not take more than one inhalation per day. Take your following amount at your usual time.
If you take too much Trelegy Ellipta, call your healthcare provider or go to the nearest hospital emergency room right away if you have any unusual symptoms, such as worsening shortness of breath, chest pain, increased heart rate, or shakiness.
It’s important to be aware of the potential side effects of Trelegy Ellipta. Here’s a breakdown of common and serious side effects, along with some guidance:
Common Side Effects:
Serious Side Effects:
Rinsing your mouth with water after each use can help prevent oral thrush.
If you experience mild side effects like headaches or coughs, they may subside as your body adjusts to the medication.
Trelegy Ellipta and certain other medicines may interact with each other. This may cause serious side effects. Especially tell your healthcare provider if you take:
Do not use other medicines that contain a LABA or an anticholinergic for any reason. Ask your healthcare provider or pharmacist if any of your other medicines are LABA or anticholinergic medication.
Get your Trelegy at a significant discount through our secure online service. We source directly from reputable Canadian pharmacies, providing Health Canada-approved medications that are identical in quality to those in the US. Enjoy the convenience of home delivery and the assurance of dealing with a legitimate source. Order online or call us for assistance.
At Insulin Outlet, we ensure the safety and quality of our medications, meeting all federal legislation standards. Your order will be dispatched from a reputable licensed Canadian pharmacy, guaranteeing the highest standards of reliability and authenticity. This medication has been approved by Health Canada (Canada's FDA) and is identical to what you would receive in the US, only with Canadian labeling and packaging. We do not sell counterfeit or unauthorized medications. Our discounted medications are shipped directly from Canada to your doorstep, providing a fast and secure delivery experience. To conveniently buy Trelegy online at a significant discount, place your order above or call us at 1-888-238-0872.
